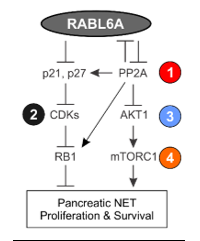
Drug targetable pathways and biomarkers of Neuroendocrine Tumors (NETs)
NETs are rare tumors arising from the diffuse neuroendocrine system, develop in both children and adults, and are dramatically increasing in incidence and prevalence in the US. They are slow growing, difficult to treat malignancies that are resistant to traditional anti-cancer chemotherapies. NET patients often survive longer after diagnosis than people with other cancers, but once tumors become advanced there are few effective treatments. Steve Jobs and Aretha Franklin are two well-known people who had pancreatic NETs. The Quelle lab is part of a large team of investigators at the University of Iowa whose translational studies of NETs are supported by a ‘NET Specialized Program of Research Excellence (SPORE) in Human Cancer’ grant funded by the National Cancer Institute (https://uihc.org/neuroendocrine-spore-overview). Our project aims to identify clinically relevant therapeutic targets that control pancreatic NET (PNET) proliferation and survival. We also seek to establish genetic and proteomic biomarker tests that discriminate NET type and prognosis. These studies, done in collaboration with many clinical and basic science colleagues (particularly Drs. Ben Darbro, Terry Braun, Andrew Bellizzi and James Howe) have uncovered novel pathways driving PNET pathogenesis that may offer new therapeutics options for patients.